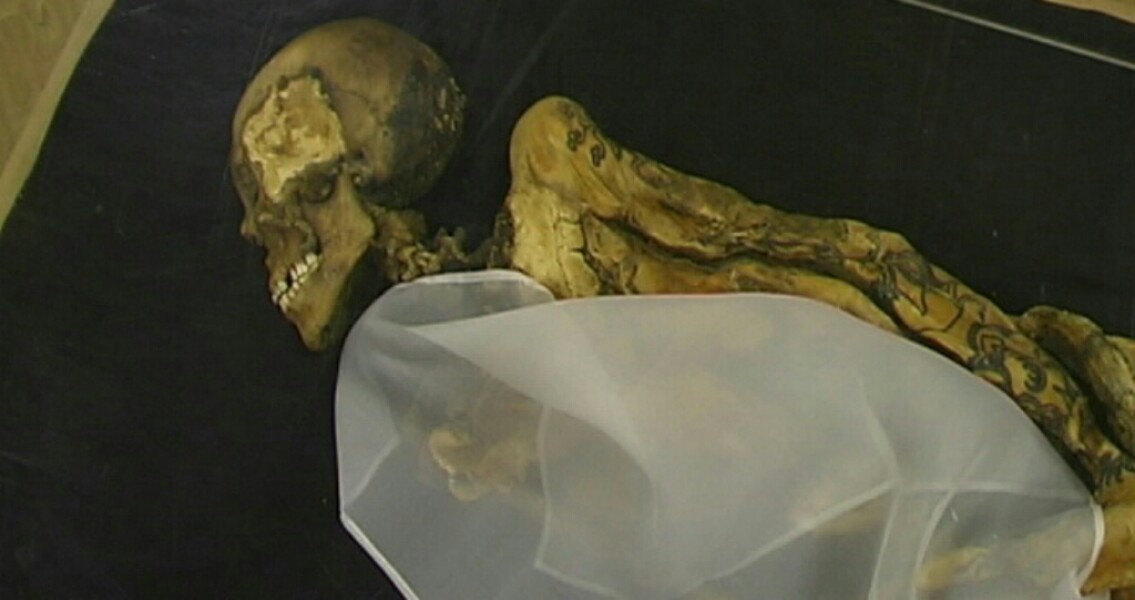
Environmental Research Letters argues that the level of distortion caused by anthropogenic emissions can actually be precisely identified. He believes this can be done by measuring the carbon isotope C13. For a long time radiocarbon dating has been widely considered the best method for determining the age of organic materials. It is believed to be accurate for objects with a biological origin dating to up to 50,000 years old. All living things contain a mixture of carbon 14 and carbon 12 atoms, with the ratio between the two remaining close to constant. Although carbon 14 atoms are constantly decaying, they are replaced by new carbon 14 atoms at a constant rate. As soon as an organism dies, the carbon 14 atoms stop being replaced. As the carbon 14 decays the ratio between it and carbon 12 starts to change, because the amount of carbon 12 remains constant. As scientists know the half life of carbon 14 (5,700 years), they can work out the age of any dead organism by comparing the ratio of carbon 12 to carbon 14 in it with the ratio in a living thing. Last year however, a problem was found which threatened the long term reliability of the radiocarbon dating method. Scientists discovered that humanity’s use of fossil fuels such as oil, natural gas and coal was significantly altering the balance between the two carbon isotopes. They concluded radiocarbon dating could become inaccurate within decades. “If the global emissions of fossil carbon dioxide continue to rise in the near future, as is proposed by the business-as-usual scenario of the Intergovernmental Panel on Climate Change, the results of our age readings of new organic material in 2050 will be identical to 1000-year-old samples. In 2150 new samples will appear to be the same age as 3000-year-old carbon, and in extreme cases even the same as 4300-year-old material,” explained Dr Peter Köhler, geoscientist at the Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research (AWI). “This means that fresh samples, for example of a tree felled within the next century, when measured using radiocarbon dating will appear to be the same age as wood that is several thousand years old.” This process is known as the Suess effect, named after the physicist Hans E. Suess. Köhler, the author of the new study, set himself the task of finding a way to determine if material had been distorted, and how significantly. “If we determine the 13C value of the sample as well as using the radiocarbon method, we can find out whether the age is trustworthy. That’s because the 13C value tells us whether the carbon of the sample has been affected by fossil carbon dioxide,” he said in a press release from the Alfred Wegener Institute. Put simply, the factors that affect carbon 14 also affect carbon 13. Carbon 13 however, is a stable isotope like carbon 12. “The burning of oil, coal and gas not only changes the 14C signal in the atmosphere, but also the stable 13C signal. This means: If my measurement shows a distorted 13C signal, then this also tells me that the 14C-based age has been affected by fossil carbon. If, on the other hand, my 13C signal is within the expected range, then the fossil carbon has not had an effect and the 14C dating method shows the correct age,” Using a range of computer models which simulate global carbon cycles, Köhler calculated the Suess effect of carbon 13 and carbon 14 up to the year 2500. He then tested a number of scenarios where mankind managed to reduce the amount of carbon dioxide. In all of the scenarios, he found that any distortions in the dating method could be detected and calculated with the 13C Suess effect. ]]>